Calculate The Number Of Molecules In 14G Of Carbon Dioxide
Calculate The Number Of Molecules In 14G Of Carbon Dioxide. 6) the salt copper sulfate can be made by reacting copper carbonate with dilute sulfuric acid. The total energy of the satellite in terms of g0, the value of.
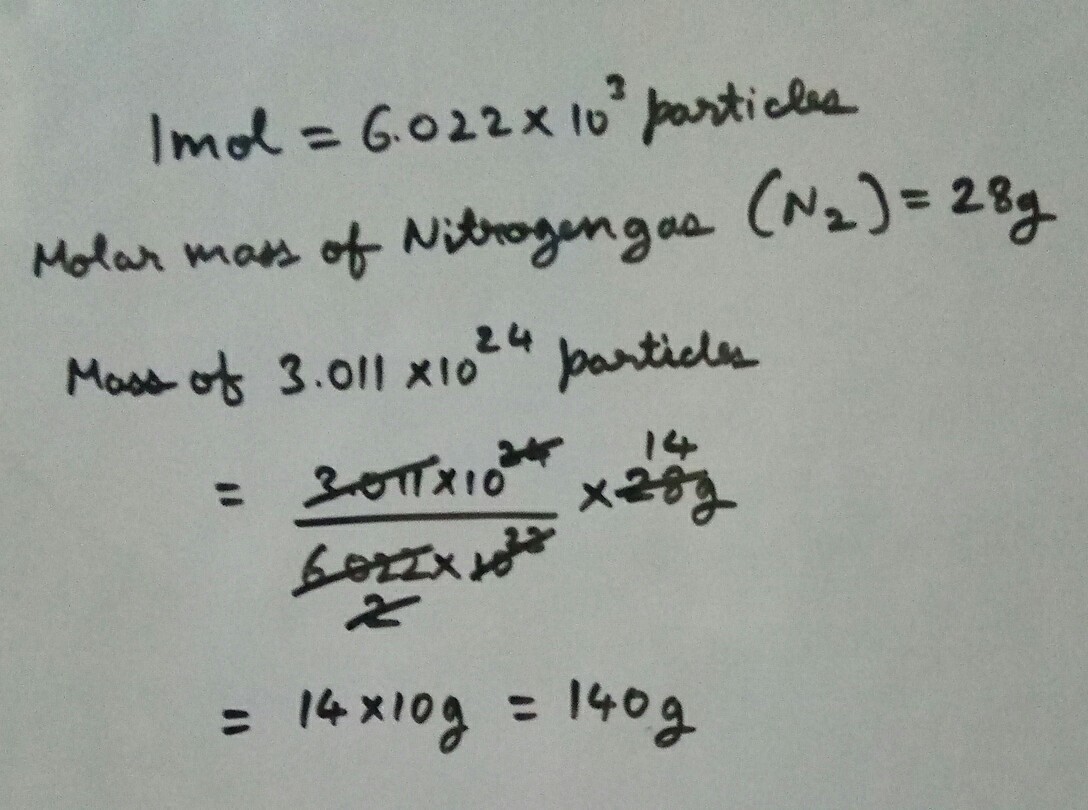
Where n a = avocado number ≡ 6.022 ×10−23 ⋅ mol−1. 140g 28.01 g mol = 5.00mol. 6) the salt copper sulfate can be made by reacting copper carbonate with dilute sulfuric acid.
220 ⋅ G 44.01 ⋅ G ⋅ Mol−1 ×N A = 5.00 ⋅ Mol.
Of moles = given mass/ molar mass so, no. The molar mass of carbon monoxide ( co) is 28.01 g/mol. Calculate the mass in grams for each of the following.
And So Avogadro's Number Of Carbon Dioxide Molecules Have This Mass.
Molar mass of nitrogen molecule n2 is 28g. 6.45×10 22 molecules of dinitrogen trioxide, n2o3n2o3. We know the molar mass of co2(g) is 44.01 ⋅ g ⋅ mol−1.
Of Atoms In 14G Of Nitrogen = 0.5.
6) the salt copper sulfate can be made by reacting copper carbonate with dilute sulfuric acid. Solution for calculate the number of molecules in 17.8 grams of carbon dioxide (co2)? Finding the number of moles question.
1.82×10 24 Atoms Of Krypton, Krkr.
O 2.11 x 1023 molecules o 1.51 x 1023 molecules o 2.23 x 1023 molecules o… The number of moles of hydrogen iodide present at equilibrium are : 8 gms of carbon monoxide is:
And We Take The Product Of The Quotient.
5.00mol ×6.022 ×1023mol−1 = 3.01 ×1024. Calculate the number of moles containing each of the following. Then multiply by avogadro's number to obtain the number of molecules:
Comments
Post a Comment